C01
Development of molecular tools for manipulating and studying memory engrams
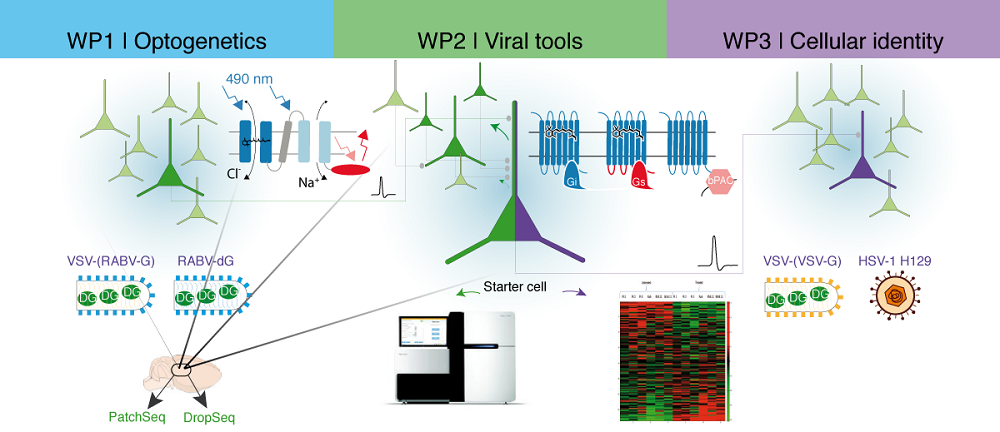
The C01 project provides customized optogenetic, viral and transcriptome analysis tools to investigate the mechanisms of memory consolidation. We will support other projects by extensive consultation and knowledge transfer, and develop novel tools specifically designed to enable state-of-the-art memory research. In the area of optogenetics we will tailor recently published actuators and sensors for project-specific implementations and develop new optogenetic tools for photocontrol of intracellular signaling cascades involving Ca2+, cAMP and G-protein signaling, enabling novel avenues for modulating cellular memory formation. For optimized viral gene transfer and circuit analysis, we improve existing retrograde tracers by reducing cytotoxicity and develop novel, herpesvirus-based tracers that promise efficient low toxicity anterograde trans-synaptic tracing for neuronal circuit analysis. Finally, we provide support for applying PatchSeq, DropSeq, and 10xVisum single cell omics platforms to identify cellular components of memory engrams and to investigate which gene expression patterns contribute to the formation of these engrams. Our work will also be beneficial for the neuroscience community outside the CRC as we make our tools available worldwide through DNA repositories and our viral core facility.
Image Schematic overview of the three work packages of the C01 project – optogenetics, viral tools, and transcriptome readouts. Image by Thorsten Trimbuch, Johannes Vierock, Benjamin Rost.
Graphical Abstract
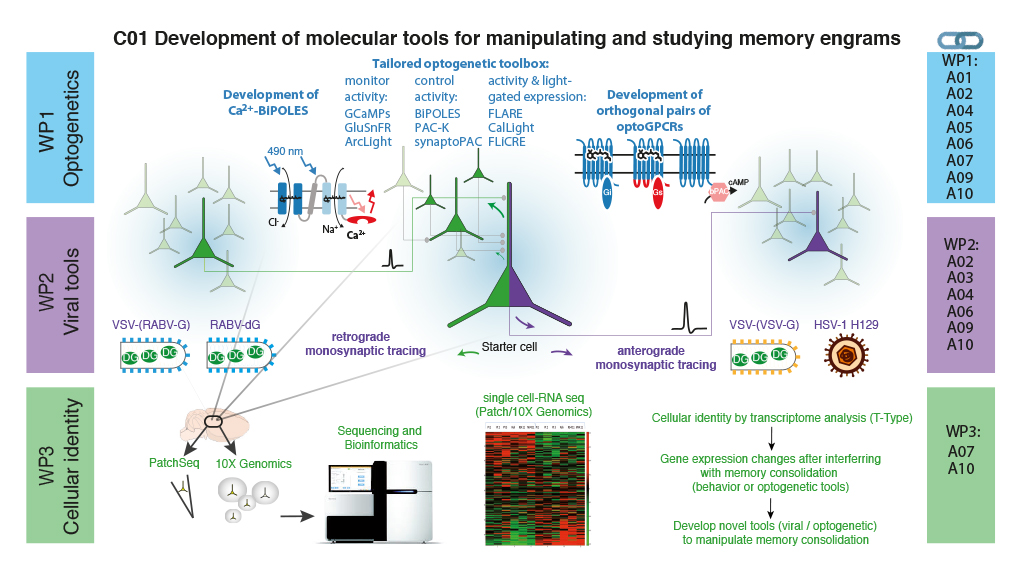
Graphical Abstract: WP1 will customize and validate existing optogenetic tools and develop two new classes of optogenetic actuators that allow for light-gated Ca2+ influx while avoiding strong membrane depolarization, and optical bidirectional control of intracellular G-proteins and 2nd messengers. WP2 will develop viral systems for anterograde and retrograde trans-synaptic tracing for neuronal circuit analysis. WP3 will provide analytical tools for deciphering the transcriptional landscape of single cells at high-resolution. Collaborations for the WPs are indicated on the right.
Team
-

Prof Dr Christian Rosenmund
Charité-Universitätsmedizin Berlin
Head Rosenmund Lab, C01 PI
-

Prof Dr Peter Hegemann
Humboldt-Universität zu Berlin
Head Hegemann Group - C01 Alumnus PI
-

Dr Benjamin Rost
Charité – Universitätsmedizin Berlin │ DZNE
C01 PI
-

Dr Johannes Vierock
Charité – Universitätsmedizin Berlin │ NWFZ
Head Subcellular Optogenetics Lab, C01 PI
-

Dr Thorsten Trimbuch
Charité-Universitätsmedizin Berlin
Head, Viral Core Facility (VCF)
-

Dr Anisha Dayaram
Charité-Universitätsmedizin Berlin
C01 Postdoc
-

Dr Beatriz Rebollo-Gonzalez
Charité-Universitätsmedizin Berlin
C01 Associate Postdoc
-

Dr Yinth Andrea Bernal
Humboldt-Universität zu Berlin
C01 Associate Postdoc
-

Linda-Jhelisa Tillert
Charité-Universitätsmedizin Berlin
C01 Associate PhD (AG Vierock)
-

Aikaterini Salivara
Charité – Universitätsmedizin Berlin
C01 PhD (AG Rost)
-

Niklas Meyer
Charité – Universitätsmedizin Berlin
C01 PhD (AG Vierock)
-

Lukas Faiss
Deutsches Zentrum für Neurodegenerative Erkrankungen
C01 PhD (AG Rost)
-

Seulkee Yang
Charité – Universitätsmedizin Berlin
C01 PhD (AG Rosenmund)
-

Dr Matthias Broser
Humboldt-Universität zu Berlin
C01 Alumnus
-

Claire Cooper
Charité-Universitätsmedizin Berlin
A01 PhD (C01 alumna)
Publications
Reaction Dynamics in the Chrimson Channelrhodopsin: Observation of Product-State Evolution and Slow Diffusive Protein Motions
Ivo H.M. van Stokkum, Yusaku Hontani, Johannes Vierock, Benjamin S. Krause, Peter Hegemann, John T.M. Kennis
J Phys Chem Lett. 14(6):1485-1493 (2023)
All-optical closed-loop voltage clamp for precise control of muscles and neurons in live animals
Amelie C. F. Bergs, Jana F. Liewald, Silvia Rodriguez-Rozada, Qiang Liu, Christin Wirt, Artur Bessel, Nadja Zeitzschel, Hilal Durmaz, Adrianna Nozownik, Holger Dill, Maëlle Jospin, Johannes Vierock, Cornelia I. Bargmann, Peter Hegemann, J. Simon Wiegert & Alexander Gottschalk
Nat Commun. 14:1939 (2023)
Genetic identification of novel medullary neurons underlying congenital central hypoventilation syndrome
Ke Cui, Yiling Xia, Abhisarika Patnaik, Elijah D. Lowenstein, Eser Göksu Isik, Adrian L. Knorz, Laura Airaghi, Michela Crotti, Michèle Studer, Filippo M. Rijli, Hans G. Nothwang, Luis R. Hernandez-Miranda
Sci Adv. 10(25):eadj0720 (2024)
Simultaneous spectral illumination of microplates for high-throughput optogenetics and photobiology
Arend Vogt, Raik Paulat, Daniel Parthier, Verena Just, Michal Szczepek, Patrick Scheerer,
Qianzhao Xu, Andreas Möglich, Dietmar Schmitz, Benjamin R. Rost and Nikolaus WengerBiol Chem (2024)
Layer 1 of somatosensory cortex: an important site for input to a tiny cortical compartment
Julia M T Ledderose, Timothy A Zolnik, Maria Toumazou, Thorsten Trimbuch, Christian Rosenmund, Britta J Eickholt, Dieter Jaeger, Matthew E Larkum, Robert N S Sachdev
Cereb Cortex. 33(23):11354-11372 (2023)
Efficient generation of a self-organizing neuromuscular junction model from human pluripotent stem cells
Alessia Urzi, Ines Lahmann, Lan Vi N. Nguyen, Benjamin R. Rost, Angélica García-Pérez, Noemie Lelievre, Megan E. Merritt-Garza, Han C. Phan, Gary J. Bassell, Wilfried Rossoll, Sebastian Diecke, Severine Kunz, Dietmar Schmitz & Mina Gouti
Nat Commun. 14(1):8043 (2023)
A bistable inhibitory OptoGPCR for multiplexed optogenetic control of neural circuits
Jonas Wietek, Adrianna Nozownik, Mauro Pulin, Inbar Saraf-Sinik, Noa Matosevich, Daniela Malan, Bobbie J. Brown, Julien Dine, Rivka Levy, Anna Litvin, Noa Regev, Suraj Subramaniam, Eyal Bitton, Asaf Benjamin, Bryan A. Copits, Philipp Sasse, Benjamin R. Rost, Dietmar Schmitz, Peter Soba, Yuval Nir, J. Simon Wiegert and Ofer Yizhar
Nat Methods (2024)
WiChR, a highly potassium-selective channelrhodopsin for low-light one- and two-photon inhibition of excitable cells
Johannes Vierock, Enrico Peter, Christiane Grimm, Andrey Rozenberg, I-Wen Chen, Linda Tillert, Alejandro G. Castro Scalise, Marilù Casini, Sandra Augustin, Dimitrii Tanese, Benoît C. Forget, Rémi Peyronnet, Franziska Schneider-Warme, Valentina Emiliani, Oded Béjà, Peter Hegemann
Sci Adv 8(49):eadd7729 (2022)
Calcium-permeable channelrhodopsins for the photocontrol of calcium signalling
Rodrigo G. Fernandez Lahore, Niccolò P. Pampaloni, Enrico Peter, M.-Marcel Heim, Linda Tillert, Johannes Vierock, Johannes Oppermann, Jakob Walther, Dietmar Schmitz, David Owald, Andrew J. R. Plested, Benjamin R. Rost, Peter Hegemann
Nat Commun. 13:7844 (2022)
Optogenetics at the presynapse
Benjamin R. Rost, Jonas Wietek, Ofer Yizhar & Dietmar Schmitz
Nat Neurosci 25, 984–998 (2022)
Far-red absorbing rhodopsins, insights from heterodimeric rhodopsin-cyclases
Matthias Broser
Front Mol Biosci. 8:806922 (2022)
Creating detailed metadata for an R-shiny analysis of circadian behavior sequence data
Julien Colomb, York Winter
Front Neurosci (in press) 2021
BiPOLES is an optogenetic tool developed for bidirectional dual-color control of neurons
Johannes Vierock, Silvia Rodriguez-Rozada, Alexander Dieter, Florian Pieper, Ruth Sims, Federico Tenedini, Amelie C. F. Bergs, Imane Bendifallah, Fangmin Zhou, Nadja Zeitzschel, Joachim Ahlbeck, Sandra Augustin, Kathrin Sauter, Eirini Papagiakoumou, Alexander Gottschalk, Peter Soba, Valentina Emiliani, Andreas K. Engel, Peter Hegemann , J. Simon Wiegert
Nat Commun. 12(1):4527 (2021)
Efficient optogenetic silencing of neurotransmitter release with a mosquito rhodopsin
M. Mahn, I. Saraf-Sinik, P. Patil, M. Pulin, E. Bitton, N. Karalis, F. Bruentgens, ..... D. Schmitz, A. Lüthi, B. R. Rost, J. S. Wiegert and O. Yizhar
Neuron. 109(10):1621-1635.e8 (2021)
Somatostatin interneurons activated by 5-HT2A receptor suppress slow oscillations in medial entorhinal cortex
Roberto de Filippo, Benjamin R Rost, Alexander Stumpf, Claire Cooper, John J Tukker, Christoph Harms, Prateep Beed, Dietmar Schmitz
eLife. 10:e66960 (2021)

SynaptoPAC, an optogenetic tool for induction of presynaptic plasticity
Silvia Oldani, Laura Moreno‐Velasquez, Lukas Faiss, Alexander Stumpf, Christian Rosenmund, Dietmar Schmitz, Benjamin R Rost
J Neurochem. 156(3):324-336 (2021)
Layer 6b is driven by intracortical long-range projection neurons
Timothy A. Zolnik, Julia Ledderose, Maria Toumazou, Thorsten Trimbuch, Tess Oram, Christian Rosenmund, Britta J. Eickholt, Robert N.S. Sachdev, Matthew E. Larkum
Cell Rep. 30(10):3492-3505.e5 (2020)
Generation of sharp wave-ripple events by disinhibition
Roberta Evangelista, Gaspar Cano, Claire Cooper, Dietmar Schmitz, Nikolaus Maier and Richard Kempter
J Neurosci. 40(41):7811-7836 (2020)
Membrane bridging by Munc13-1 is crucial for neurotransmitter release
Bradley Quade, Marcial Camacho, Xiaowei Zhao, Marta Orlando, Thorsten Trimbuch, Junjie Xu, Wei Li, Daniela Nicastro, Christian Rosenmund
eLife. 8:e42806 (2019)

